Uncharted Territory: One Man’s Story of Navigating Prostate Cancer and a Clinical Trial


Sponsored by Merck
When Euvon Jones was only 59 years old, he faced a startling diagnosis of stage IV prostate cancer that had spread to his lymph nodes and bones in his back, hip and chest. Euvon explored treatment options with his oncologist, but due to the advanced stage of his cancer, surgery, chemotherapy and radiation were deemed unsuitable. However, Euvon was eligible to participate in a clinical trial studying an investigational treatment.
Initially apprehensive, Euvon had reservations about joining a clinical trial. He was hesitant because of a lack of understanding about clinical trials, which were an “uncharted territory” for him. In fact, he didn’t know anyone who had participated in a clinical trial before. However, with education from his oncologist, the love and support of his family and his faith in God, he eventually decided to enroll. Three pivotal factors played a role in his decision:
- He trusted his oncologist because she was transparent about the clinical trial process and talked him through the risks and possible benefits to consider as he was making a decision.
- He had confidence in his oncologist’s 30 plus years of experience and knowledge about clinical trials.
- His oncologist shared that she was also impacted by prostate cancer because of her father’s diagnosis with the condition. Because of this, she was devoted to doing her best to help people with cancer receive the best care and treatment for their disease. This deepened Euvon’s confidence that he made the right choice in choosing her as his doctor.
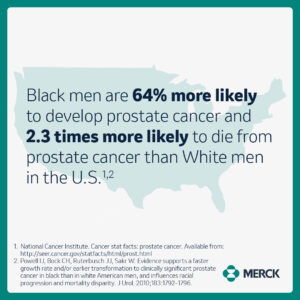 Historically, Black patients have been underrepresented in medical research and in cancer clinical trials, including those for prostate cancer.¹ This remains an issue today because Black people are at higher risk of diagnosis and death from certain cancers than people of other races.²
Historically, Black patients have been underrepresented in medical research and in cancer clinical trials, including those for prostate cancer.¹ This remains an issue today because Black people are at higher risk of diagnosis and death from certain cancers than people of other races.²
People of different ages, sexes, ethnicities, and races can respond differently to medicines, and without diverse representation in clinical trials, the effectiveness and safety of these products may not be fully understood for all groups.
After completing the clinical trial, Euvon became committed to sharing his journey with cancer, including his experience participating in a clinical trial. Euvon’s advice to others who may be facing a difficult diagnosis or complicated medical decisions is to become educated. His oncologist gave him a USB drive filled with information about the trial, which helped him understand and feel more comfortable with the clinical trial process and alleviate his concerns.
“Study, study, study,” said Euvon. “Talk with your doctor and ask questions. Become a student of your diagnosis. Become a student of what may have contributed or impacted your condition. Try to absorb and explain the process to yourself. Try to wrap your brain around the clinical trial in a way that makes sense to you. Drop the pride – it’s okay to admit that you need help.”
“It’s critical that people with cancer become involved in their treatment journey from the start. This includes asking their doctors about clinical trials they might be eligible to participate in. Diverse populations, including Black/African Americans, have been historically underrepresented in clinical trials because they are oftentimes not aware these trials are available,” said Luther T. Clark, MD, FACC, FACP, Executive Director, Global Medical and Scientific Affairs at Merck. People from diverse communities are needed to participate in clinical trials to help us understand whether investigational treatments are safe and effective in people from various backgrounds. That’s why we have a variety of initiatives in place to improve access to and diversity of our clinical trials.”
Currently, Euvon, a motivational artist, is enjoying retirement with his wife, Janet, four children and six grandchildren. He dedicates his time to his family, church and sharing his journey with prostate cancer with the wish that it will inform and inspire others to learn more about clinical trials.
For more information about clinical trial diversity, visit www.merck.com/trialdiversity.
# # #
References
- Swaby J, Kaninjing E, Ogunsanya M. African American participation in cancer clinical trials. Ecancermedicalscience. 2021 Oct 25;15:1307. doi: 10.3332/ecancer.2021.1307. PMID: 34824630; PMCID: PMC8580719.
- Centers for Disease Control and Prevention. African American People and Cancer. 2023 July 19. Accessed 2023 Dec. 5: https://www.cdc.gov/cancer/health-equity/groups/african-american.htm
- Hinata N, Fujisawa M. Racial Differences in Prostate Cancer Characteristics and Cancer-Specific Mortality: An Overview. World J Mens Health. 2022 Apr;40(2):217-227. doi: 10.5534/wjmh.210070. Epub 2022 Jan 1. PMID: 35021294; PMCID: PMC8987139.




